

32.Typically form ions with charges of +3 and +6 Use the following responses to answer questions 33-37. Measurements indicate the effective nuclear charge experienced by a 2s lithium electron is 0.43 times the. A 2s lithium electron can have 2 1s electrons between itself and the lithium nucleus.
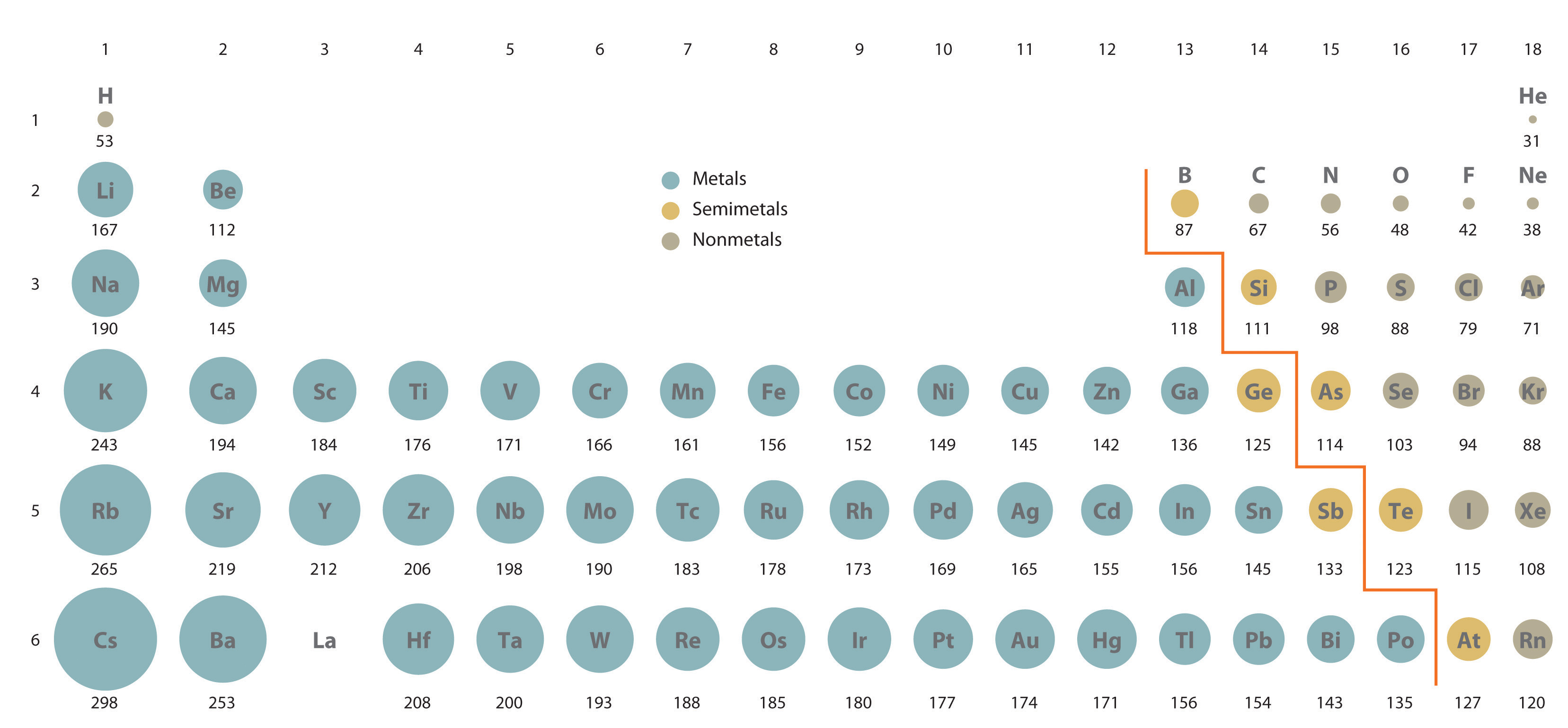
An anion is relatively larger in size than its parent atom. The shielding effect explains the trend in atomic size on the periodic table and also why valence electrons are readily removed from an atom. The atomic size of a cation will be smaller than that of the parent atom. The Ionic radius can be described as the distance between the nucleus of an ion and the outermost shell of the ion.
#Atomic size trend ap che full#
Show a reversal in the trend for first ionization energy because of shielding by full orbitals. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. The radius increases as you move down a group (column) and decreases as you move from left to right across a period (row). Show a reversal in the trend for first ionization energy because of electron-electron repulsions. The third IE, however, is over five times the previous one. Both atomic radius and ionic radius follow a trend on the periodic table. This similarity occurs because the members of a group have the same number and distribution of electrons in their valence shells. The properties and their periodic trends are described below. The second IE is twice the first, which is not a surprise: the first IE involves removing an electron from a neutral atom, while the second one involves removing an electron from a positive ion. Describe and explain the observed trends in atomic size of the elements The elements in groups (vertical columns) of the periodic table exhibit similar chemical behavior. Definition: Electronegativity Periodic trend in electronegativity Metallic character Periodic trend in metallic character Summary of the periodic trends Properties of elements generally show a periodic trend that correlates with their position in the periodic table. Why is it so much larger? Because the first two electrons are removed from the 3 s subshell, but the third electron has to be removed from the n = 2 shell (specifically, the 2 p subshell, which is lower in energy than the n = 3 shell).(g) + e^− \nonumber \] AP Chem > Unit 1 1.7 Periodic Trends 8 min read decemJeremy Kiggundu Dalia Savy Share A cool thing about the periodic table is that it is organized to demonstrate different trends and properties of elements that can be explained by the pattern of electron configurations and the presence of electron-filled orbitals. The third IE, however, is over five times the previous one. Although there are some reversals in the trend (e.g., see Po in the bottom row), atoms generally get smaller as you go across the periodic table and larger as you go down any one column.

The second IE is twice the first, which is not a surprise: the first IE involves removing an electron from a neutral atom, while the second one involves removing an electron from a positive ion. Terms in this set (18) What is the shape of the s orbital What is the shape of the p orbital What is the shape of the d orbital How many orbitals are in each sublevel Square the principal energy level to find the number of orbitals i.e. 1: Atomic Radii Trends on the Periodic Table.


 0 kommentar(er)
0 kommentar(er)
